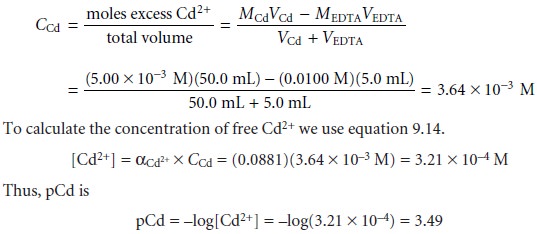
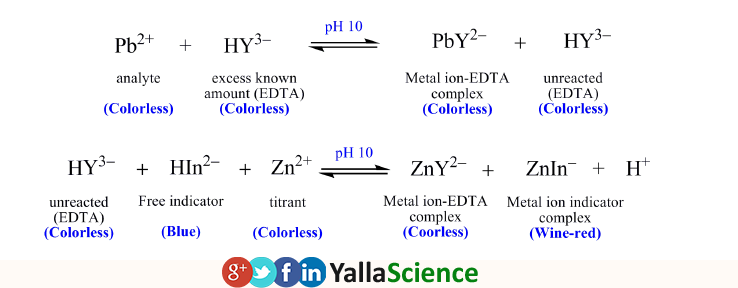
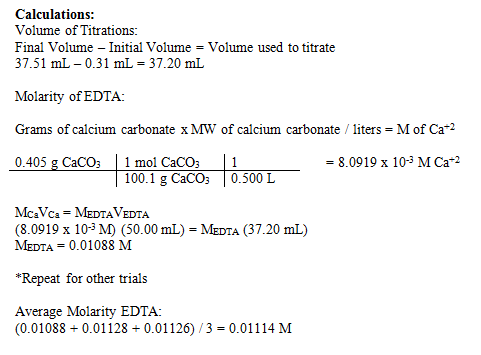
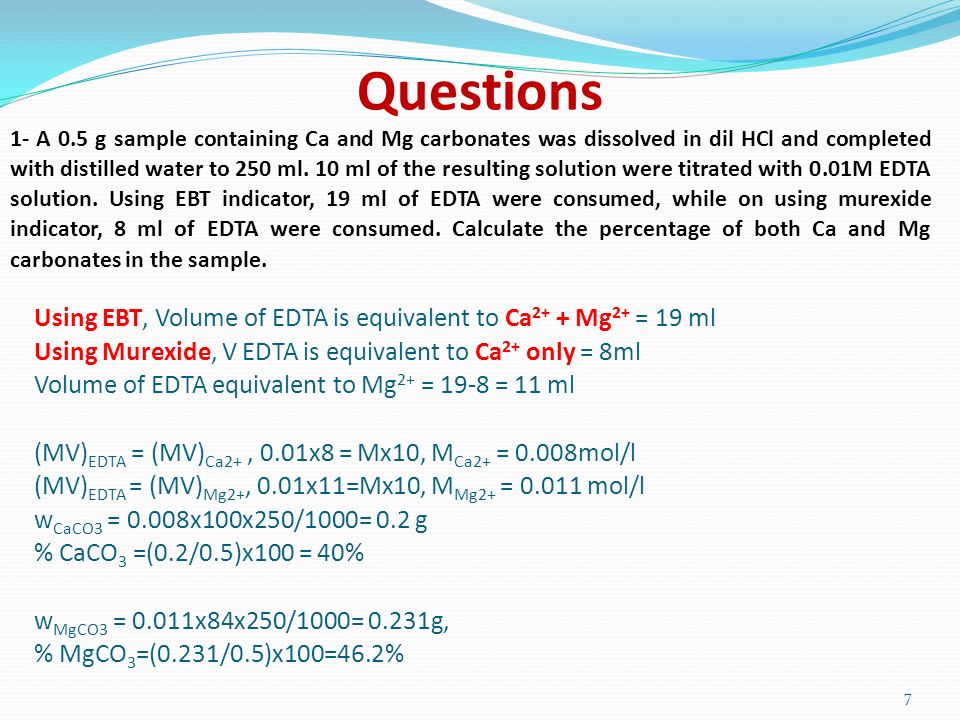
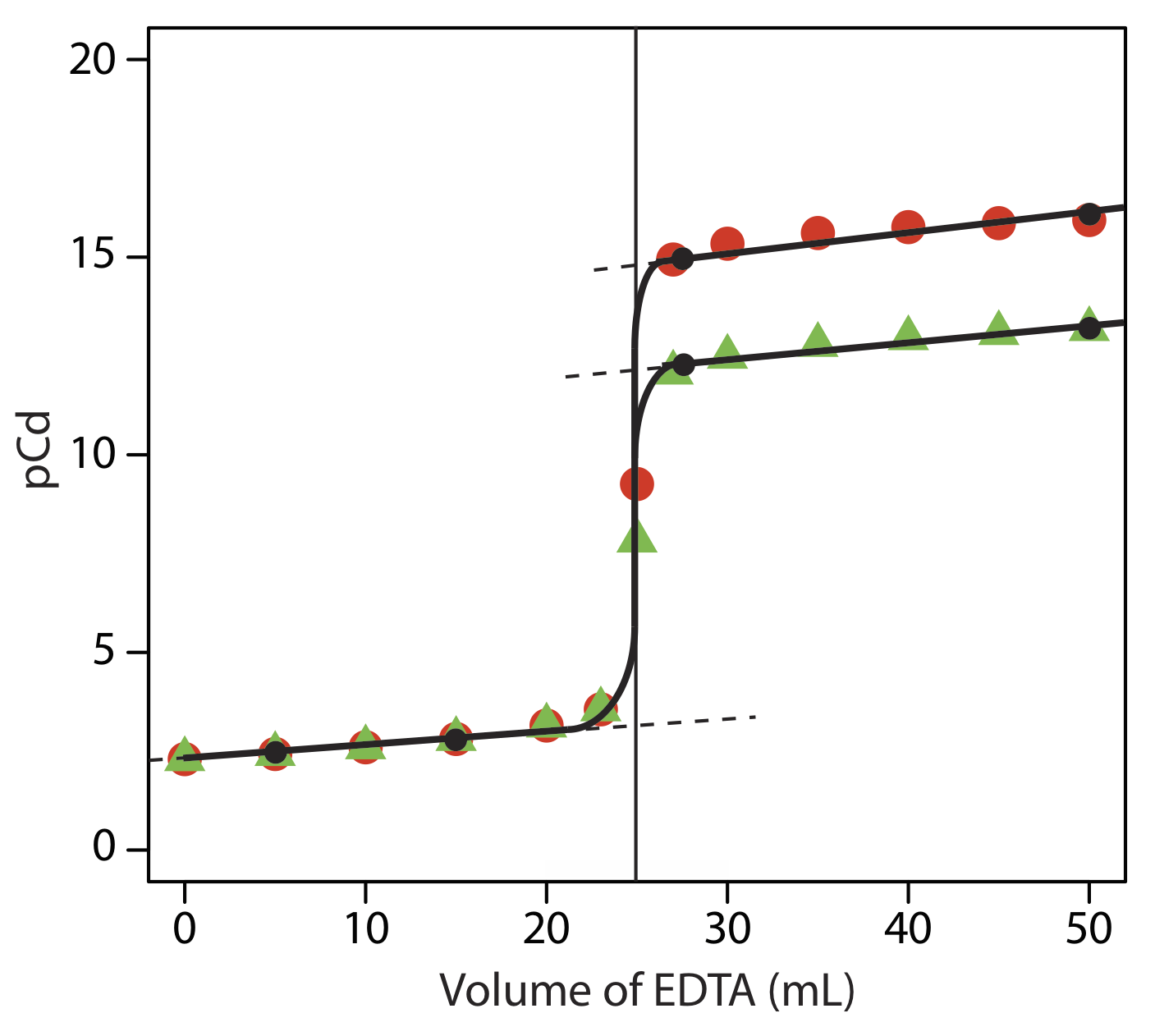
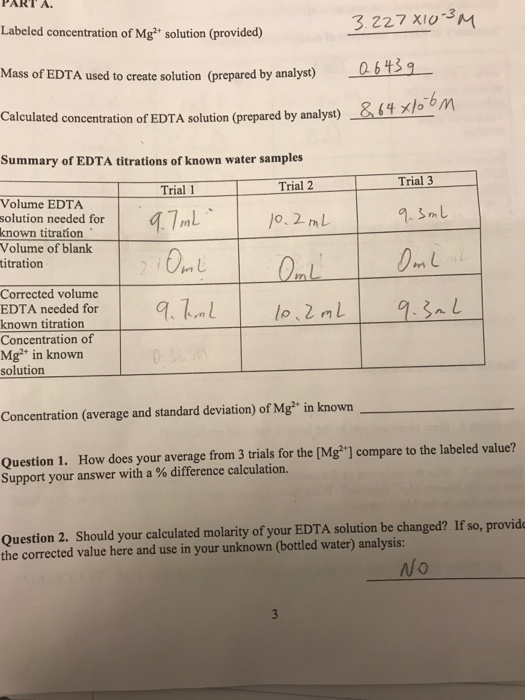
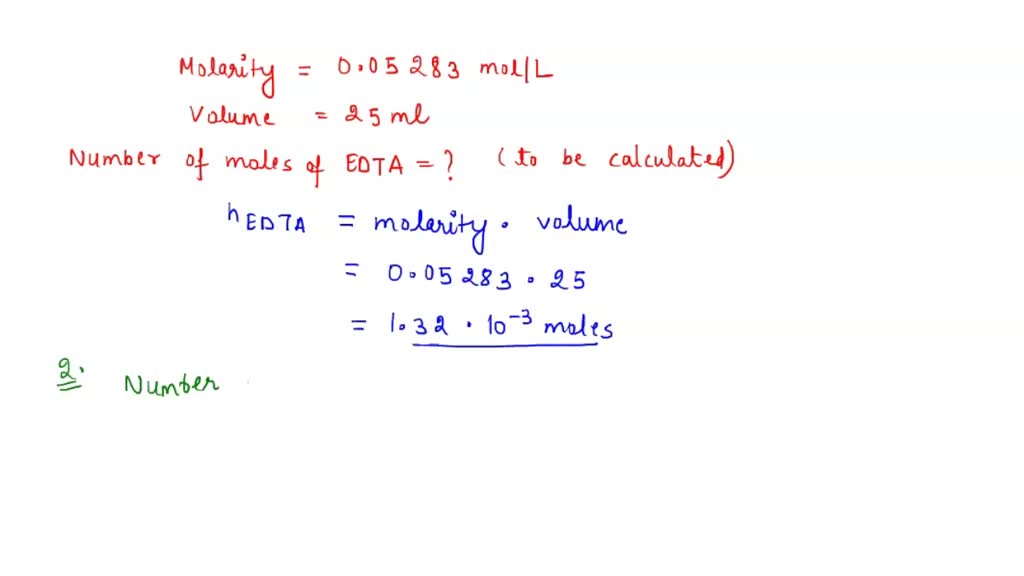Understanding Complexometric Titrations of Metal Cations with Aminopolycarboxylic Acids (EDTA and Analogs) within the frame of the Notion of Reactions between Groups of Chemical Species

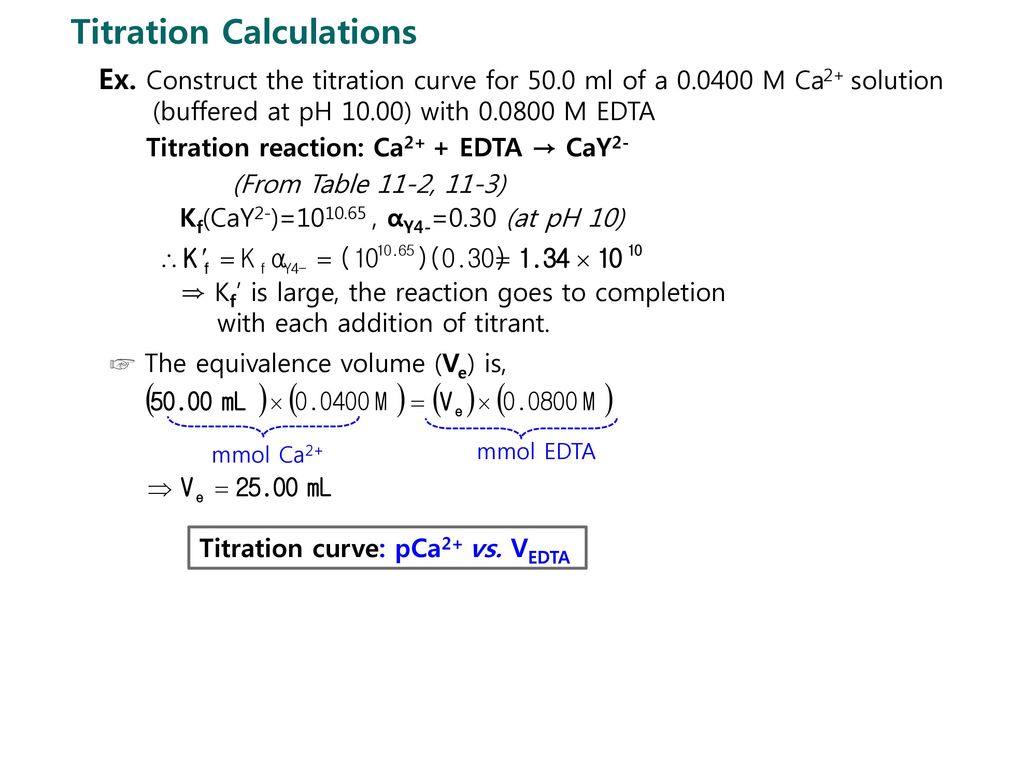
SOLVED: Standard EDTA Titration The concentration of standardized EDTA is determined by titration of 25.0 mL of 0.100 M CaClz solution by 40.0 mL EDTA. Scandium is determined by titration of Sc3+
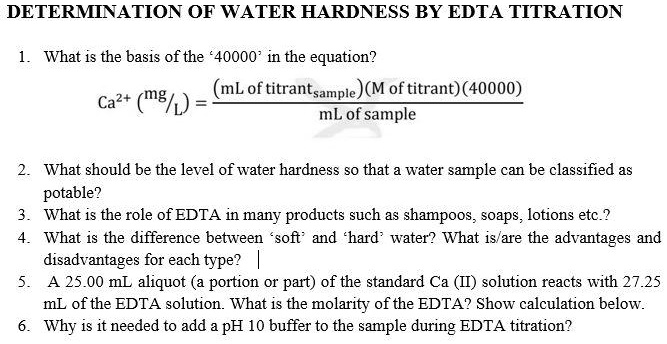
SOLVED: DETERMINATION OF WATER HARDNESS BY EDTA TITRATION What is the basis of the *40000' in the equation? (mLof titrantsample)(M of titrant)(40000) Ca2+ (mg/,) mL of sample What should be the level
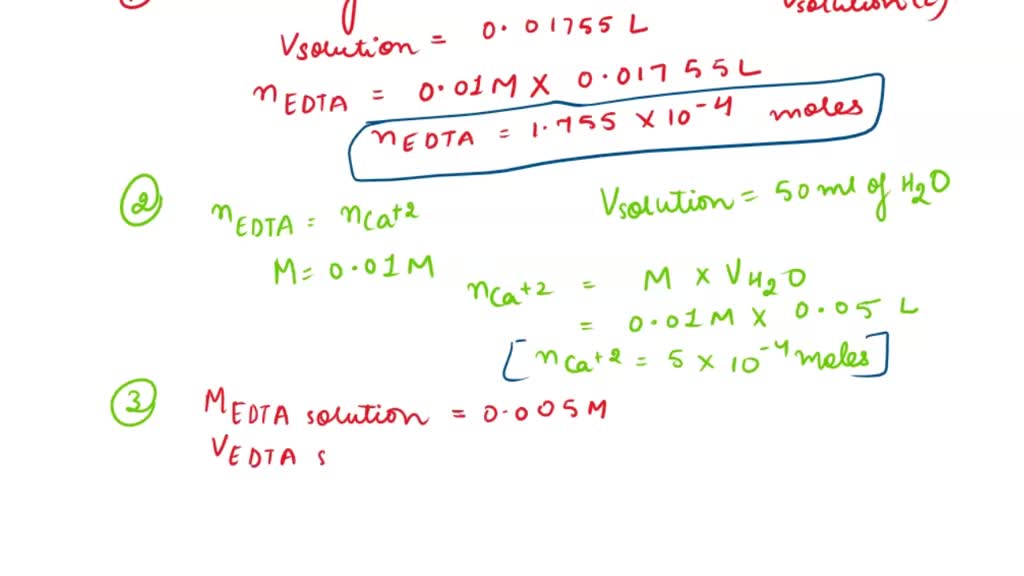
SOLVED: 1) Use the molarity of the EDTA solution and the average volumeof EDTA added to calculate the average number of moles of EDTArequired for the titration. Molarity EDTA= .01 Volume EDTA= .
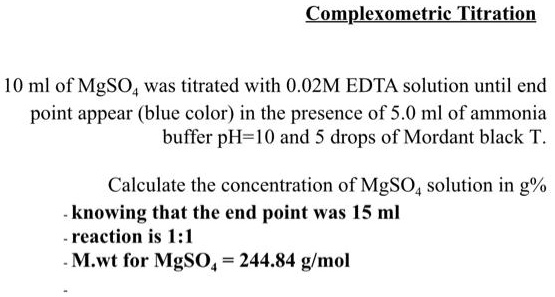
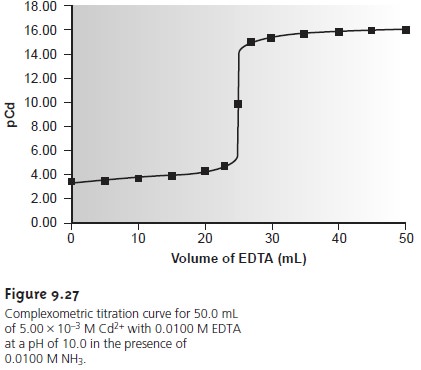
SOLVED: Complexometric Titration 10 ml of MgSO4 was titrated with 0.02M EDTA solution until end point appear (blue color) in the presence of 5.0 ml of ammonia buffer pH-I0 and 5 drops