SOLVED: A solution of hydrogen peroxide is 30.0% H2O2 by mass and has a density of 1.11 g/cm3. Calculate the molarity of the solution. Show your calculation or expalin your answer.

Calculate the molarity and molality of a 13% solution (by weight of sulphuric acid). Its density is 1.090 g/ml.
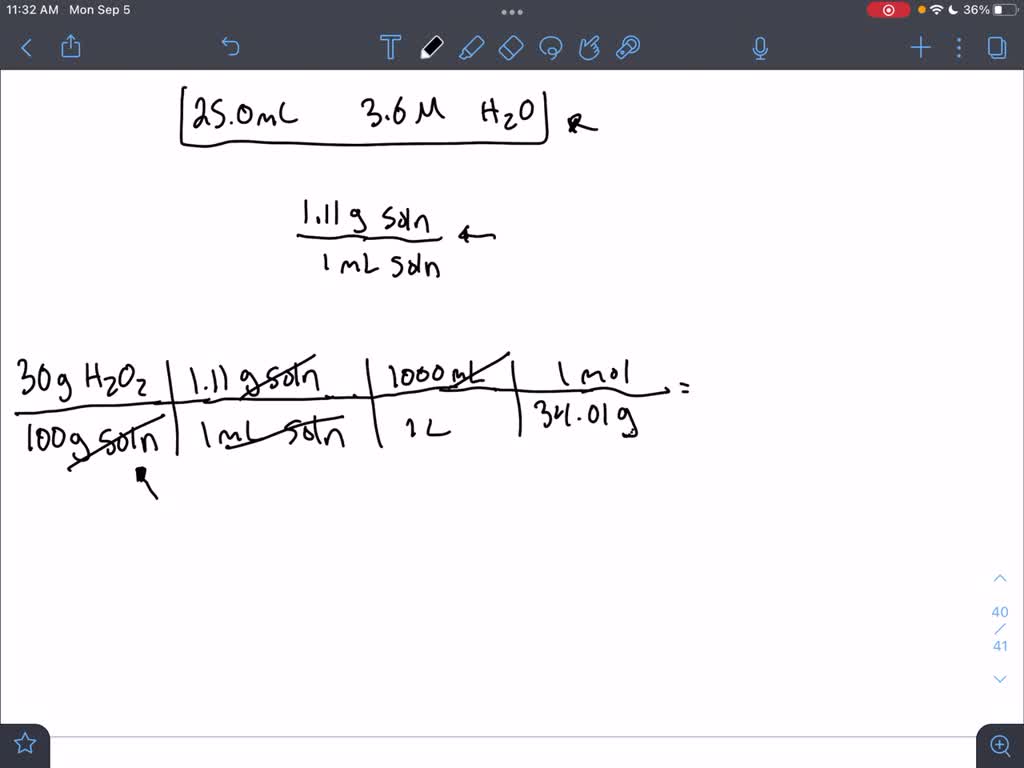
SOLVED: You are required to make up 25.0 ml of a 3.6 M solution of hydrogen peroxide (H2O2). You have at your disposal a stock solution that is 30% by weight, H2O2,

Calculator the equilibrium concentration of gaseous hydrogen peroxide over solution – Lukáš Kolík – physico-chemical calculation – working a personal webpage
For "16.8 V" H2O2 solution ( density = 0.95 g/ml), calculate : 1. Molarity 2. Molality 3. 4. 5. ppm of solute 6. mole fraction of solute



![The volume strength of 1 M H2O2 is:[Molar mass of H2O2 = 34 g mol^-1 ] The volume strength of 1 M H2O2 is:[Molar mass of H2O2 = 34 g mol^-1 ]](https://dwes9vv9u0550.cloudfront.net/images/2154655/640a9e6a-45be-4444-9242-719c87344f1c.jpg)
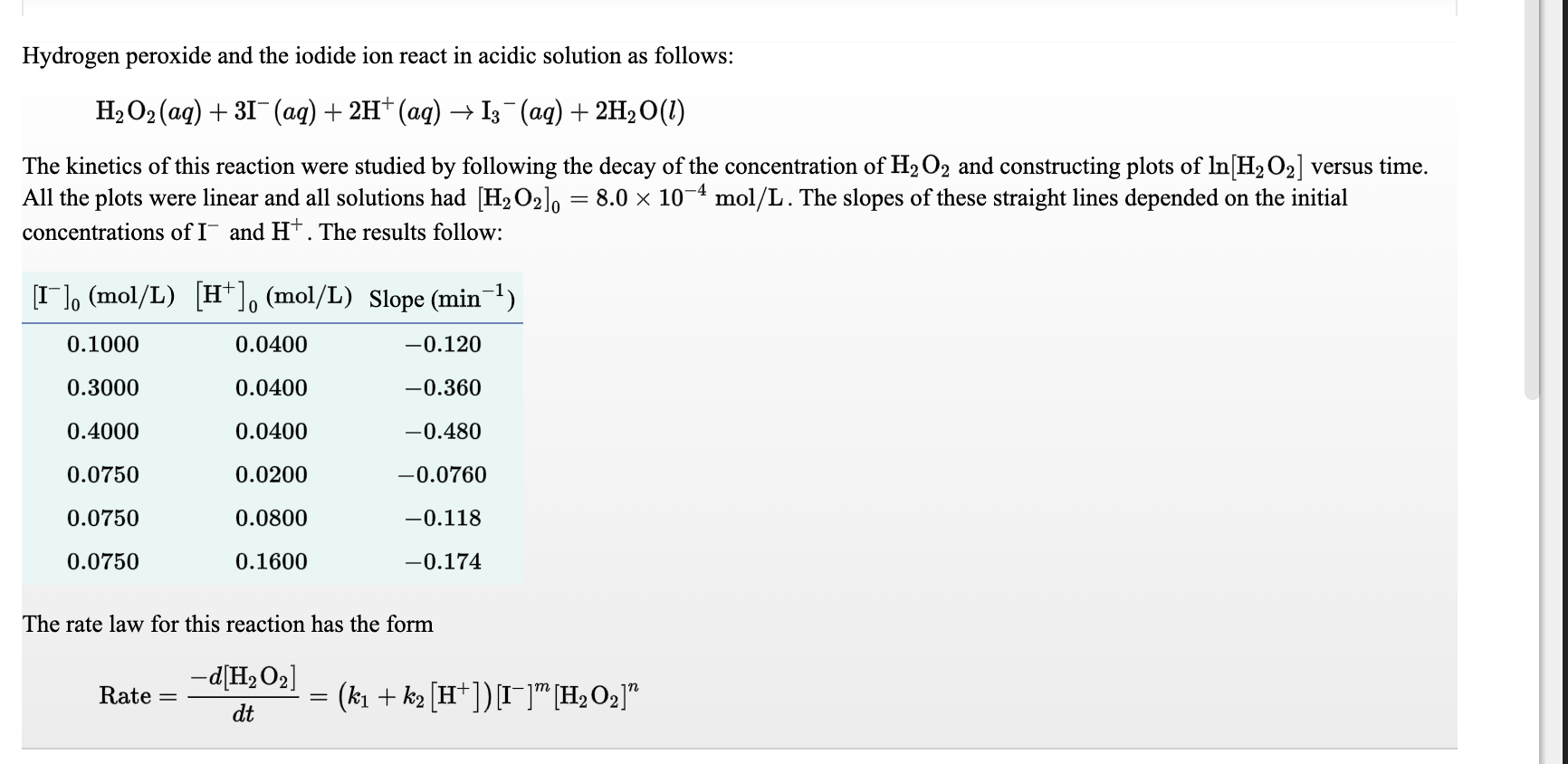


![Hydrogen Peroxide [H2O2] Molecular Weight Calculation - Laboratory Notes Hydrogen Peroxide [H2O2] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2021/11/hydrogen-peroxide-molecular-weight-calculation.jpg)