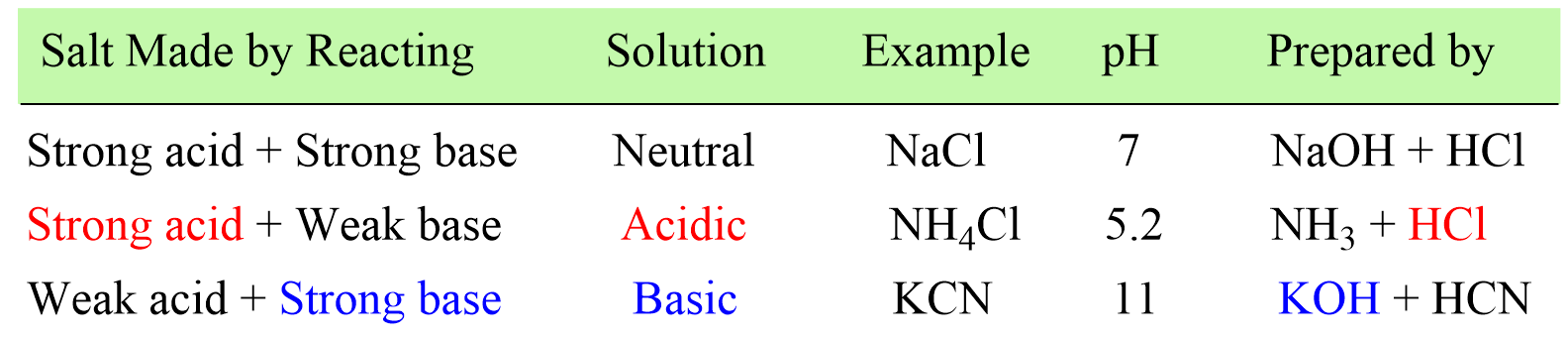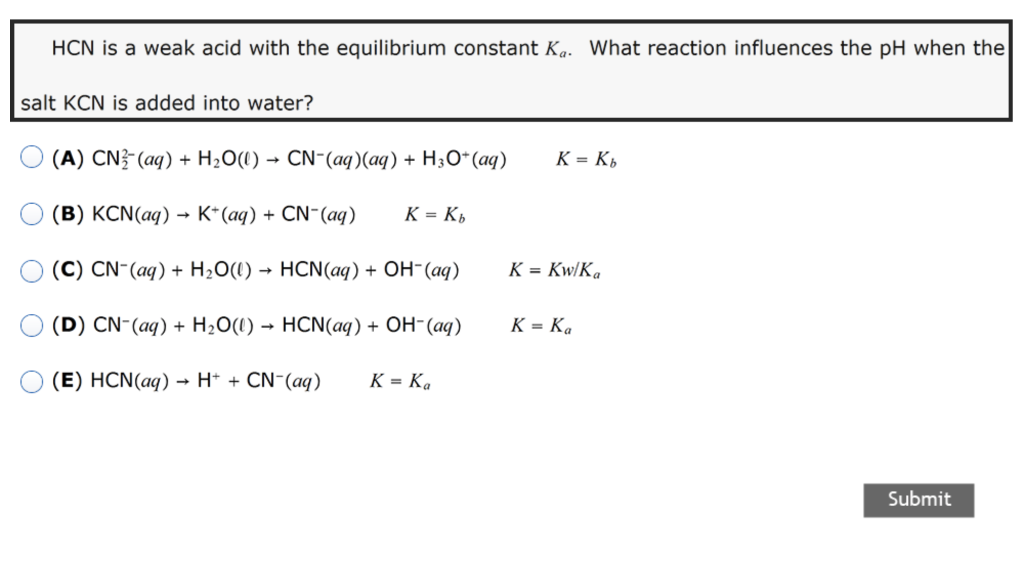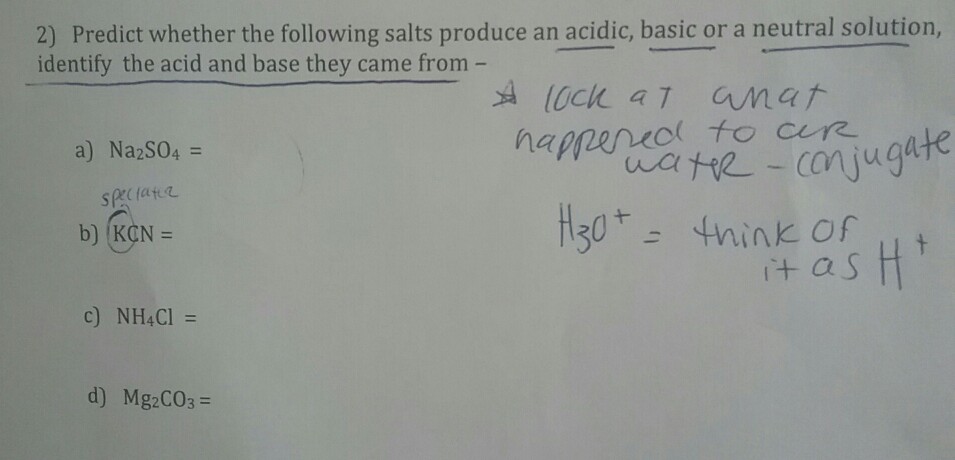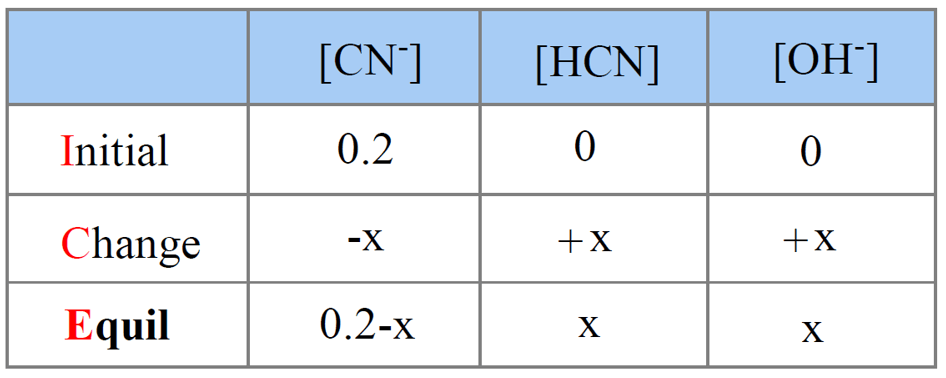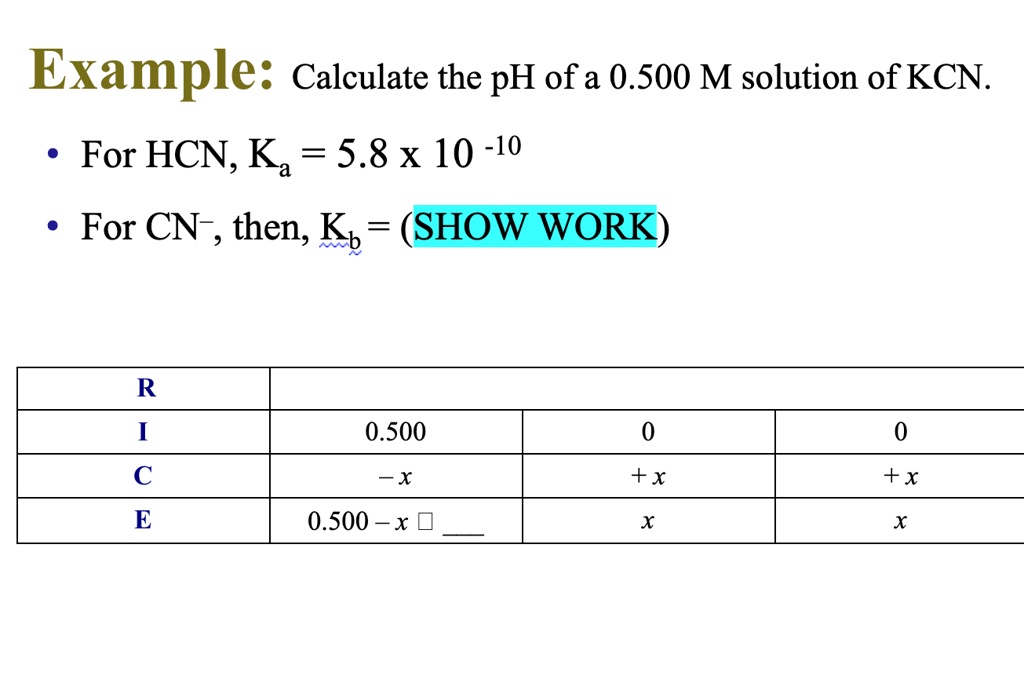
SOLVED: Example: Calculate the pH ofa 0.500 M solution of KCN For HCN, K = 5.8 x 10 -10 For CN , then, Kb (SHOW WORK) R 0.500 c E X 0.500 -x 0 X +X +X

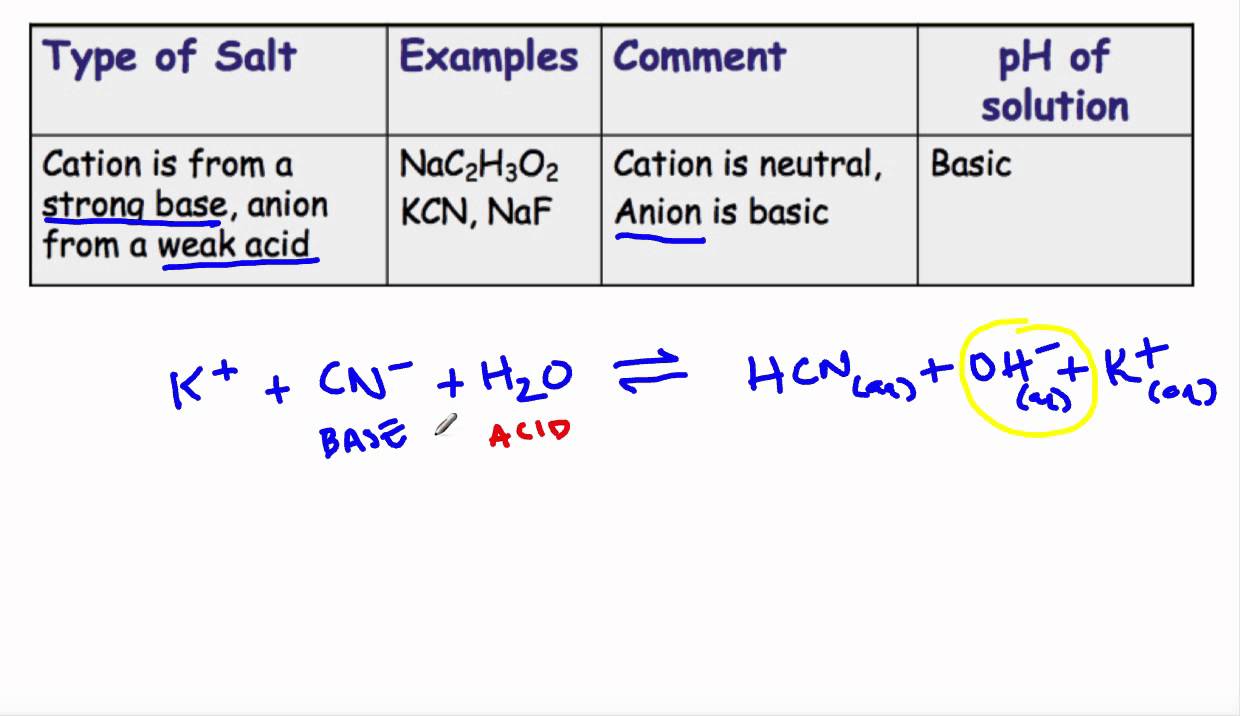
pH of salt solutions 1.Salts derived from strong acids and strong bases These consist of cations from strong bases and the anions from. - ppt download

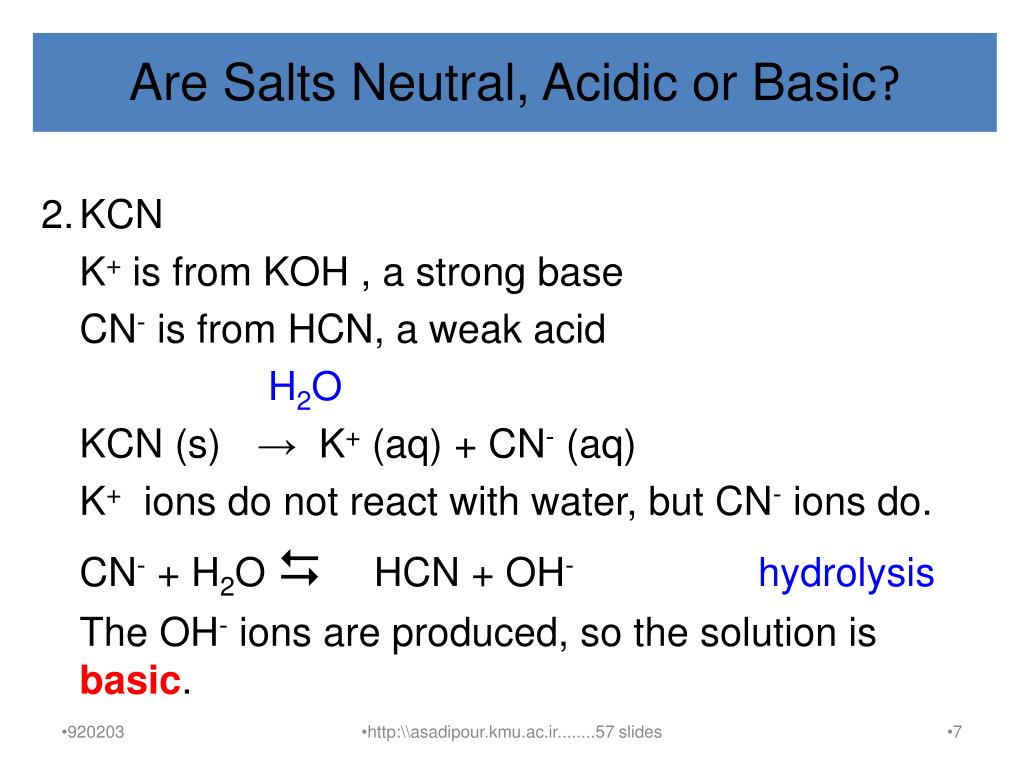
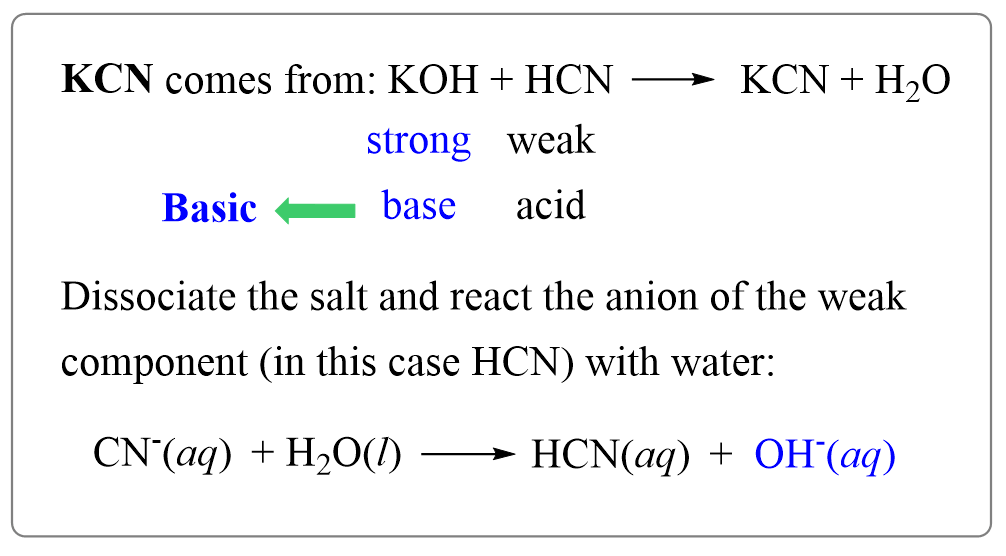
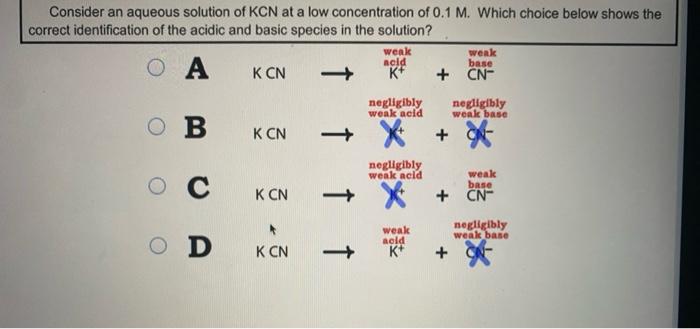
When potassium cyanide reacts with water, will the resulting solution be acidic, alkaline or neutral? Justify your answer.

Acid-Base Properties of Salts. These salts simply dissociate in water: KCl(s) K + (aq) + Cl - (aq) - ppt download
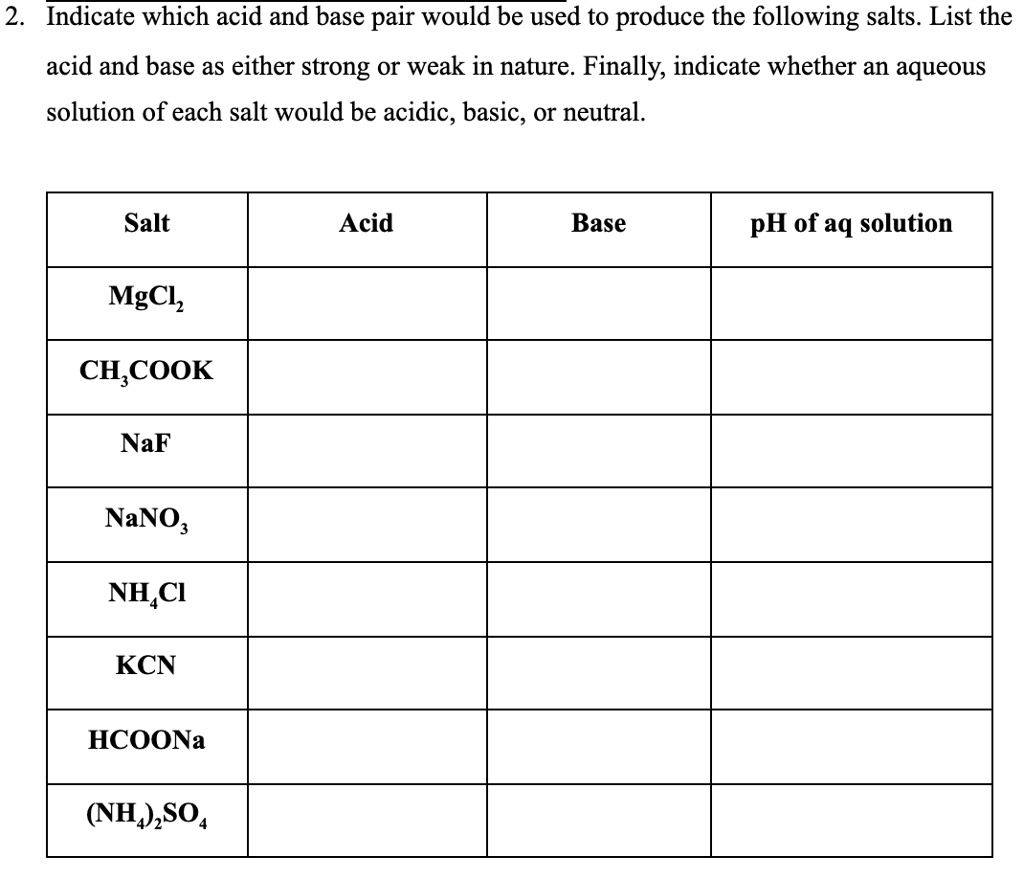
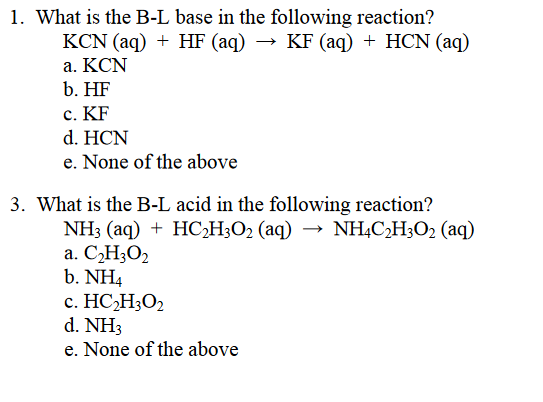
SOLVED: 2 Indicate which acid and base pair would be used to produce the following salts List the acid and base as either strong or weak in nature. Finally, indicate whether an